 English
English


Potentiometric Titration of Silver Nitrate with Sodium Chloride for Precise Analytical Measurements
Potentiometric Titration of Silver Nitrate with Sodium Chloride
Potentiometric titration is a valuable analytical technique used to determine the concentration of an unknown solution through the measurement of voltage changes in a electrochemical cell during the titration process. One classical application of this technique involves the titration of silver nitrate (AgNO3) with sodium chloride (NaCl). This titration is particularly interesting due to the formation of a precipitate, silver chloride (AgCl), which plays a critical role in the reaction dynamics and the interpretation of the results.
Reaction Overview
The reaction between silver nitrate and sodium chloride can be represented by the following equation
\[ \text{Ag}^+ (aq) + \text{Cl}^- (aq) \rightarrow \text{AgCl} (s) \]
In this reaction, silver ions from the silver nitrate react with chloride ions from the sodium chloride to form a solid precipitate of silver chloride. This reaction demonstrates the classic precipitation titration where the endpoint is indicated by a sudden change in the measured potential of the electrochemical system.
Equipment and Setup
A typical setup for potentiometric titration consists of a suitable reference electrode and an indicator electrode. In the case of silver nitrate and sodium chloride titration, a silver/silver chloride (Ag/AgCl) electrode is often used as the indicator electrode due to its ability to respond to changes in the concentration of silver ions. The reference electrode can be any stable reference electrode, such as a saturated calomel or Ag/AgCl reference.
The titration is performed by gradually adding a standard solution of sodium chloride to the silver nitrate solution while continuously monitoring the potential. The addition of NaCl leads to the formation of AgCl until all the silver ions are consumed.
Procedure
potentiometric titration of silver nitrate with sodium chloride
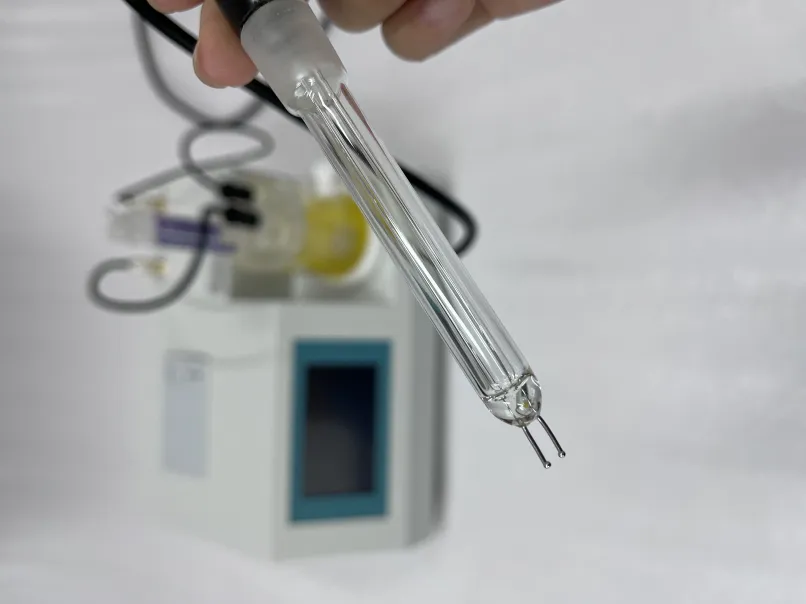
2. Calibration of Electrodes The electrodes are calibrated with standard solutions to ensure accurate potential readings.
3. Titration Process The titration is conducted by gradually adding sodium chloride to the silver nitrate solution. The voltage is recorded after each addition.
4. Data Collection The potential change is monitored continuously, and data is plotted, typically showing a sharp change in potential at the equivalence point, where all silver ions have reacted and formed AgCl.
Analysis of Results
The key to interpreting the data from this titration lies in identifying the equivalence point, which is marked by a steep slope in the potential versus volume of titrant added graph. At this point, the concentration of Ag+ ions is at its minimum, significantly affecting the potential read by the indicator electrode.
After determining the equivalence point, the concentration of the unknown silver nitrate solution can be calculated using stoichiometric relationships based on the initial concentrations and volumes used in the titration.
Conclusion
The potentiometric titration of silver nitrate with sodium chloride illustrates the effective use of electrochemical methods in analytical chemistry. By utilizing the principles of precipitation reactions and systematic monitoring of potential changes, precise quantitative analysis can be achieved. Not only does this method provide insight into the concentrations of ionic species in solution, but it also underscores the importance of electrochemistry in both educational and research settings. This method exemplifies how instrument-based techniques can enhance our understanding of chemical reactions and processes in a measurable and accurate manner.
-
Differences between open cup flash point tester and closed cup flash point testerNewsOct.31,2024
-
The Reliable Load Tap ChangerNewsOct.23,2024
-
The Essential Guide to Hipot TestersNewsOct.23,2024
-
The Digital Insulation TesterNewsOct.23,2024
-
The Best Earth Loop Impedance Tester for SaleNewsOct.23,2024
-
Tan Delta Tester--The Essential Tool for Electrical Insulation TestingNewsOct.23,2024





