 English
English


Testing the Acidity Levels in Transformer Oil for Optimal Performance and Maintenance Standards
Acidity Test for Transformer Oil Importance and Methodology
Transformer oils play a crucial role in the efficient operation of electrical transformers. These oils serve not only as insulators but also as coolants, helping to dissipate heat produced by the electrical components. Over time, various factors such as moisture, oxygen, and impurities can lead to the degradation of transformer oil, which can significantly impact the transformer's performance and longevity. One of the critical tests performed on transformer oil is the acidity test, which helps determine the oil's condition and suitability for continued use.
Importance of the Acidity Test
The acidity of transformer oil is a key indicator of its oxidation stability and overall condition. As transformer oil ages, it undergoes physical and chemical changes, many of which can lead to an increase in acidity. This rise in acidity is primarily due to the formation of organic acids as a byproduct of the oil’s oxidation process. High acidity levels can result in the following issues
1. Corrosion Increased acidity can lead to the corrosion of the metal parts of the transformer, including windings and structural components. This can significantly reduce the life expectancy of the equipment and necessitate costly repairs or premature replacement.
2. Insulation Breakdown The formation of acidic compounds can impair the oil's insulating properties. This breakdown can lead to electrical failures, short circuits, or even catastrophic transformer fires.
3. Altered Thermal Properties Degraded oil may lose its ability to effectively dissipate heat, leading to overheating and reduced operational efficiency.
Given these potential issues, monitoring the acidity of transformer oil is essential for maintaining effective transformer operation and preventing unscheduled outages.
acidity test for transformer oil
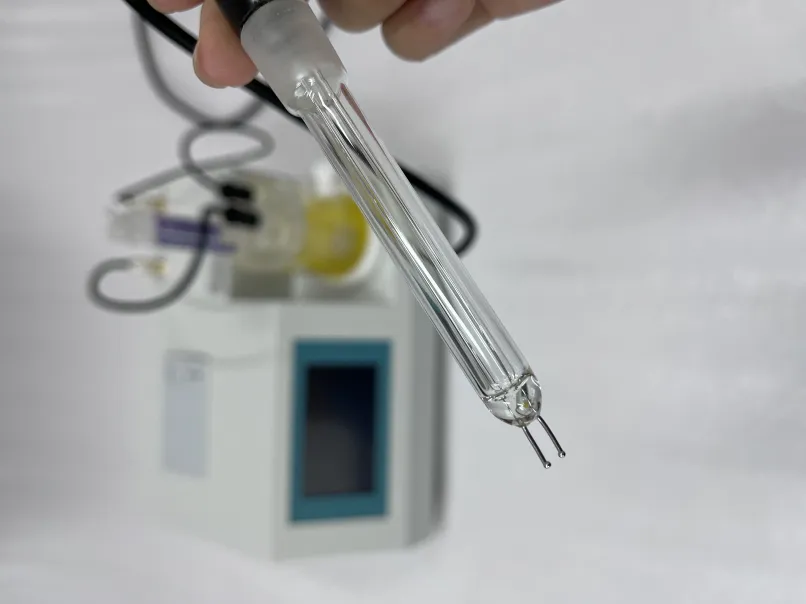
Methodology of the Acidity Test
The acidity test for transformer oil typically involves titration to determine the total acid number (TAN), which quantifies the amount of acidic substances present in the oil. The following steps outline the standard procedure
1. Sample Collection A representative sample of transformer oil is collected, ensuring it is free from contaminants. Proper handling and storage of the sample are vital to avoid any alterations before testing.
2. Preparation for Titration The oil sample is usually dissolved in a solvent, such as isopropanol, to facilitate the acid detection. This step is crucial as it prepares the oil for accurate measurement.
3. Titration Process A standardized solution of sodium hydroxide (NaOH) is slowly added to the oil sample using a burette. The addition continues until a stable endpoint is reached, indicated by a color change, often using a suitable pH indicator.
4. Calculating TAN The total amount of NaOH used is measured, and from this, the total acid number is calculated using specific formulas. The TAN is usually expressed in milligrams of KOH per gram of oil (mg KOH/g).
5. Interpretation of Results The resulting TAN value is compared against predetermined thresholds to assess the oil's condition. A TAN value below a certain level is generally considered acceptable, while higher values may indicate the need for oil replacement or purification.
Conclusion
In conclusion, the acidity test is an indispensable tool for the maintenance and management of transformer oil. By regularly performing this test, operators can detect potential problems early, implement necessary maintenance actions, and ensure the reliability and longevity of electrical transformers. Maintaining the acidity levels within acceptable limits is vital not only for the efficient operation of transformers but also for minimizing the risk of catastrophic failures and subsequent financial losses.
-
Differences between open cup flash point tester and closed cup flash point testerNewsOct.31,2024
-
The Reliable Load Tap ChangerNewsOct.23,2024
-
The Essential Guide to Hipot TestersNewsOct.23,2024
-
The Digital Insulation TesterNewsOct.23,2024
-
The Best Earth Loop Impedance Tester for SaleNewsOct.23,2024
-
Tan Delta Tester--The Essential Tool for Electrical Insulation TestingNewsOct.23,2024





